Revision of the Pharmaceutical Machinery Law (Part 3)
This is the revised column (3) on the Act on Drug Machines. This time, as an amendmentLegalization of "pioneering examination designation system" and "conditional early approval system"I will explain about.
1. Background of amendments
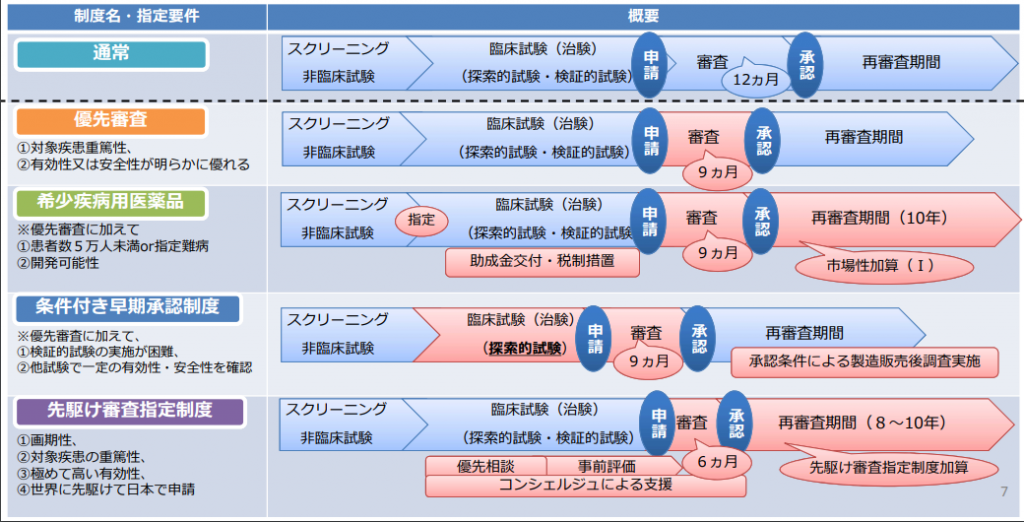
The “pioneering examination designation system” and “conditional early approval system” are preferential treatment for approval examination of pharmaceutical products. Such incentives have been expanded since the introduction of the orphan drug and orphan medical device designation system in the revision of the Pharmaceutical Affairs Law in 1993. The current preferential treatment system for approval examination of pharmaceutical products is as follows.
Preferential treatment system related to approval review system for pharmaceuticals, etc.
From the materials of the 2nd Pharmaceutical and Medical Device System Subcommittee in 2018
The “Prior Examination Designation System” was introduced in 2015, and in order to commercialize innovative drugs, innovative medical devices, etc. ahead of the rest of the world, drugs and medical treatments that are expected to show significant effectiveness in the early clinical trial stage The purpose is to shorten the time until approval is obtained by giving priority to the specified devices, and to provide the most advanced drugs and medical devices to patients as soon as possible. When the products are covered by this system, pharmaceutical companies can receive priority consultation, preliminary evaluation, and preferential treatment with a total examination period of 6 months.
The "conditional early approval system" was introduced in 2017, and it is difficult or difficult to carry out confirmatory clinical trials (evaluation trials of efficacy/safety by a large number of patients) due to the small number of patients, etc. For pharmaceuticals and medical devices that require a long period of time, we aim for early commercialization by making it a condition for approval, such as reconfirming the efficacy and safety after manufacturing and sales. For regenerative medicine products, since cells use cells and the quality is non-uniform and it takes a long time to collect and evaluate the data for confirmation of effectiveness, a conditional and time-limited approval system has been introduced since 2013.
The priority examination and orphan drug system have already been legislated, but the “pioneering examination designation system” and “conditional early approval system” were operated as notifications (indicated as interpretations of laws and systems). So it was necessary to have a system based on the law. The new system is as follows.
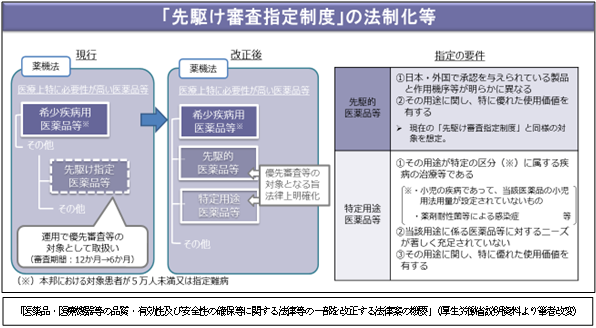
2. Legalization of the "pioneering examination designation system"
The pioneer designated drugs, etc. that are targeted in the trial implementation of the pioneering examination designation system currently operated by notification are classified and designated as "pioneer drugs, etc." and "special use drugs, etc."
“Pioneer drugs, etc.” are drugs, medical devices, etc., whose mechanism of action is clearly different from those of drugs approved in Japan/foreign countries. In addition, “special-purpose drugs, etc.” are drugs for which there is a high medical need, such as drugs for which no pediatric dosage is prescribed, and drugs that are significantly unsatisfied. Both "pioneering medicines" and "special use medicines" are prioritized over other medicines, medical devices, etc. for examination or investigation.
For "Special Purpose Drugs", necessary funds will be secured and tax measures will be taken to promote testing and research.
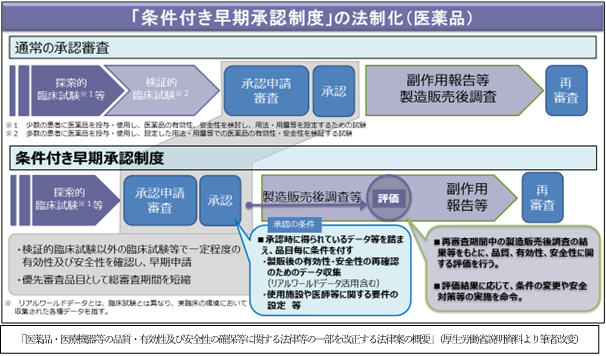
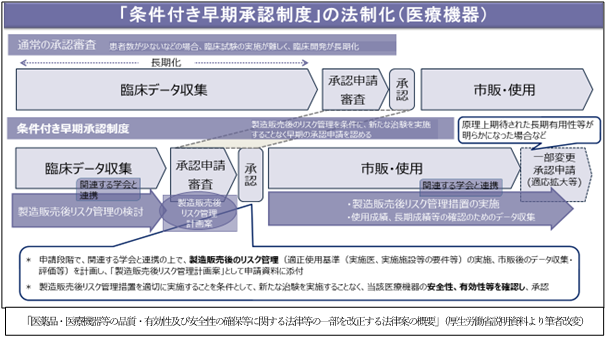
3. Legalization of "conditional early approval system"
The purpose is to secure patients' access to drugs and medical devices that have a particularly high medical need for serious and poorly effective treatments, but the contents of the system differ between drugs and medical devices. ..
With regard to pharmaceutical products, we confirm the effectiveness and safety to a certain extent and approve them by applying approval conditions to those for which confirmatory clinical trials are difficult and require a long period of time. As a condition for that, data collection is required to reconfirm the efficacy and safety after manufacturing and sales, but it is also permitted to use real world data (data collected in clinical practice). .. Although real-world data is in the verification stage, it will significantly change the method of clinical evaluation of pharmaceuticals as it becomes evaluation data for approval. In addition, approval conditions regarding facilities used and doctors are also imposed. After the manufacture and sale, the results obtained from the post-marketing survey etc. defined by the approval conditions will be evaluated, and according to the evaluation results, the conditions will be changed and safety measures will be ordered.
For medical devices, existing clinical data are evaluated for new clinical trials that are difficult to perform due to the small number of patients, risk management measures after manufacturing and sales, and data collection and evaluation after marketing. It is approved by adding such things as approval conditions. Prompt approval is expected by not conducting new clinical trials. After manufacturing and sales, it is required to implement the risk management measures specified as approval conditions.
This time, I explained the legalization of the "pioneering examination designation system" and "conditional early approval system". This legislation is scheduled to come into effect in September of this year. From the next time onward, we will continue to explain each revision.
Author: Tokuya Okanouchi
All rights reserved for this column.

Tokuya Okanouchi (CDI Medical Co., Ltd.)
Completed Graduate School of Pharmaceutical Sciences, Shizuoka Prefectural University, Completed Law School at Toin University of Yokohama, and completed Business School at the University of Massachusetts Lowell. Doctor (Pharmacy), Doctor of Law, MBA (Master of Business Administration)
After working at the Ministry of Health, Labor and Welfare, the National Hospital Organization, the Pharmaceuticals and Medical Devices Agency, the National Institute of Health Sciences, the Ministry of the Environment, the Ministry of Justice, and Kanagawa Prefecture.