Regarding the revision of the Pharmaceutical Machinery Law (Part 4)
This is a revised column (4) on the Pharmaceutical Machinery Act. This time, as a revised matter"Introduction of approval system according to the characteristics of medical devices"I will explain about.
1. Background of amendments
A medical device is a machine or device that is used for diagnosis, treatment or prevention of human or animal diseases and is intended to affect the structure or function of the body, and is defined by a Cabinet Order. There are a wide variety of medical devices, including scalpels, surgical scissors, tweezers, stents, pacemakers, MRIs, and surgical robots. In addition, it has characteristics different from those of pharmaceutical products, such as the effect being affected by the ability and skill of the user. Some products have been improved and improved, and especially for medical devices that use programs, changes have been remarkably repeated after marketing. Furthermore, the development of medical devices that constantly change their performance after marketing, such as medical devices that utilize AI (artificial intelligence), and the improvement of medical devices that use data collected after marketing are progressing. ..
Conventionally, regarding continuous improvement of medical devices, depending on the degree of impact of the change on the product, approval of some changes requiring clinical trials, approval of some changes that can be evaluated in non-clinical trials, and notification of minor changes The procedure has been taken. However, repeating the procedure for each continuous improvement is laborious and time-consuming, and has the problem of delaying patient access to the latest medical devices. Therefore, in order to realize prompt patient access to such medical devices, further regulations based on the characteristics of medical devices are required.
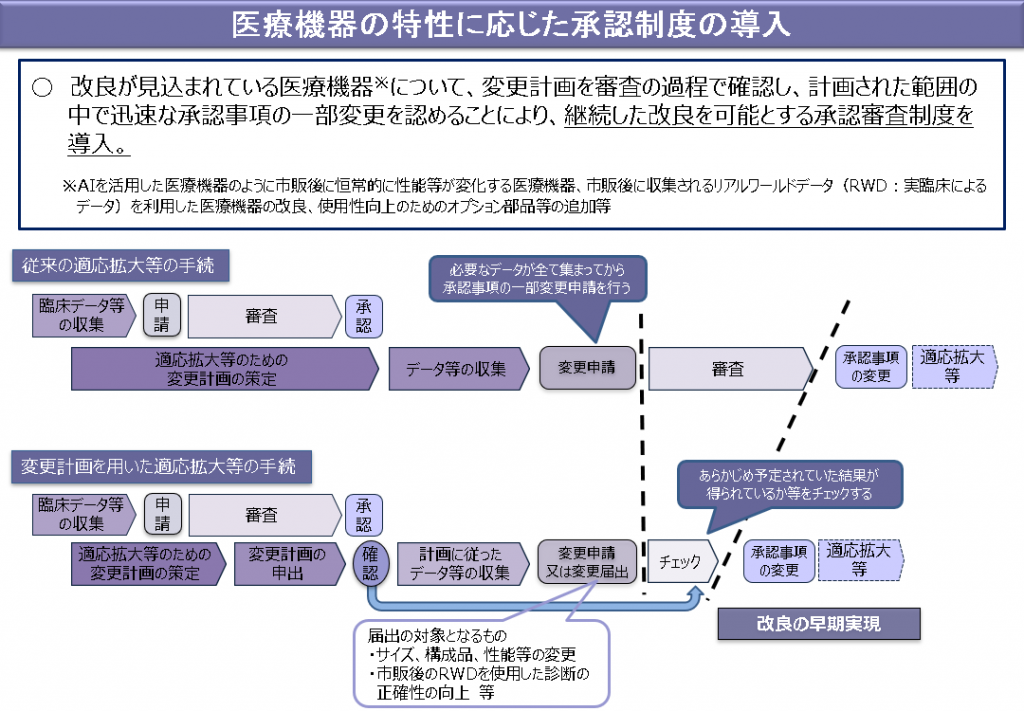
2. Contents of "Introduction of approval system according to the characteristics of medical devices"(See the outline below)
For medical devices that are expected to be improved (including in-vitro diagnostic drugs), an approval review system will be introduced to enable continuous improvement. Those who have obtained approval for medical equipment, etc. can receive confirmation from the Minister of Health, Labor and Welfare regarding plans regarding changes in performance, manufacturing methods, etc., among approved items. In addition, the person who received the confirmation shall make the change by the number of days specified by the Minister of Health, Labor and Welfare on the day when the change according to the plan (limited to the change of the manufacturing method and other changes specified by the Ordinance of the Ministry of Health, Labor and Welfare). When notified, it is not necessary to obtain approval for the change.
On the other hand, the Minister of Health, Labor and Welfare, in the case of an application for approval pertaining to changes in accordance with the plan that received the confirmation (except for changes in manufacturing methods and other changes specified by the Ordinance of the Ministry of Health, Labor and Welfare), the Instead of conducting a survey on effectiveness or safety, it is possible to conduct a survey on whether the change is in accordance with the plan.
In addition, on August 31, the Ordinance of the Ministry of Health, Labor and Welfare, which provided detailed procedures for this new system ("Act on Securing Quality, Effectiveness, and Safety of Pharmaceuticals, Medical Devices, etc., etc." Ministerial Ordinance on Development of Related Ministerial Ordinances Due to Enforcement" (Ministry of Health, Labor and Welfare Ordinance No. 155, August 31, 1993).
This ministerial ordinance mainly stipulates the following procedures for "introduction of an approval system according to the characteristics of medical devices".
(1) Classification of change plan confirmation application
① Medical equipment (other than artificial intelligence related technology)
② Medical equipment (artificial intelligence related technology)
③ In-vitro diagnostic drug
(2) When you can receive change confirmation
① Purpose/effect
② Shape / structure / principle
③ Raw materials
④ Performance / safety standards
⑤ How to use
⑥ Management method
⑦ Validity period
⑧ Manufacturing method
⑨ Factory
(3) When we cannot receive confirmation of change
① Changes that do not comply with the standards set by law
(2) Significant changes regarding inactivation and removal methods of pathogenic factors
(3) Changes that may have a serious impact on quality, effectiveness, and safety
(4) Standard paperwork period 30 days
Outline of introduction of approval system according to the characteristics of medical devices
"Outline of the bill to partially revise the law on ensuring the quality, effectiveness and safety of pharmaceuticals and medical devices" (modified by the author from the explanation material of the Ministry of Health, Labor and Welfare)
This time, I explained "Introduction of approval system according to the characteristics of medical devices". The first enforcement of the new system due to this revision of the law started in September. From the next time onward, we will continue to explain each revision.
Author: Tokuya Okanouchi
All rights reserved for this column.

Tokuya Okanouchi (CDI Medical Co., Ltd.)
Completed Graduate School of Pharmaceutical Sciences, Shizuoka Prefectural University, Completed Law School at Toin University of Yokohama, and completed Business School at the University of Massachusetts Lowell. Doctor (Pharmacy), Doctor of Law, MBA (Master of Business Administration)
After working at the Ministry of Health, Labor and Welfare, the National Hospital Organization, the Pharmaceuticals and Medical Devices Agency, the National Institute of Health Sciences, the Ministry of the Environment, the Ministry of Justice, and Kanagawa Prefecture.