産学連携における公的研究資金を獲得するには?
産学連携における公的研究資金を獲得するには?
医薬品、医療機器等の医療製品を開発する企業にとって、大学や公的研究機関との共同による研究開発の重要性は、オープンイノベーションの流れも受けて益々高まっているところです。この産学連携を進めていくために、国、自治体、財団法人、大学などの数多くの機関が産学連携を支援しています。その支援の幅は広く、知的財産、施設設備、人材、製品評価や経営相談など多岐に及びますが、最も重要なことは研究開発資金の確保についてです。特に、医療製品は開発が多段階で長期にわたるものも多く、その開発段階における適切な資金の確保が欠かせません。開発企業が連携していく大学の研究室には研究成果や知恵はあっても開発資金はないため、その欠如は製品開発における死活(死の谷)問題です。
このような医療製品に特有な研究開発の重要課題への対応を含め、医療の分野における研究開発を切れ目なく円滑に実用化されるよう、大学や研究機関が行う研究を支援しているのが、AMED(国立研究開発法人 日本医療研究開発機構)です。従来からあった文部科学省の科学研究費助成事業(文科研費)、厚生労働省の厚生労働科学研究費(厚労科研費)、経済産業省の事業費等を集めて、医療製品に係る研究開発をする大学や研究機関を財政的に支援するものです(AMED令和2年予算総額1,272億円、文科研費・厚労科研費の事業は各省庁で別途継続)。ここで重要なことは、文科研費や厚労科研費は大学と公的研究機関しか対象としていませんが、AMEDとなったことで産学連携による研究開発において連携する企業も支援の対象となったことです。これにより、産学連携を通じて開発企業も公的研究資金の獲得が可能となりました*。
2015年からAMEDによる研究開発事業が開始され、医療製品開発を支援する数多くの事業が公募され実施されています。私も、これらの研究開発事業に応募(申請段階)や実際の研究開発事業において関わってきました。研究申請が採択されて順調に研究開発が進んでいるものがある一方、残念ながら採択を受けられなかったものがあります(採択倍率は主に5-10倍)。公募研究事業の申請はAMEDの審査による評価を受けて、その評価が高かったものの順に予算規模の範囲の中で採択されますが、研究事業への申請のご相談などを受けていると気づく点があります。
申請段階においては、開発目的、科学的技術、研究内容、研究の実行可能性などを示してアピールします。ここでは提案する自己の研究開発が、研究として「いかに優れているか」ではなく、AMEDの主催する研究事業に「いかに適合しているか」が重要です。これを掛け違いして申請をされる方が結構多いのです。その理由は、AMEDは政府の研究事業を実施するための機関だからです。政府の研究事業は、上位機関である文部科学省、厚生労働省、経済産業省などが国の政策を策定して予算を取ります。それぞれの官庁がそれぞれの政策に合った研究事業を実施するために、医療製品の研究開発の予算はAMEDへと流れます。そこでAMEDは、その政策を実現するための研究事業を企画して、その事業の実施のために最も適合する研究開発者を募集しているのです。そのため、産学連携の支援といっても主催する研究事業を実施してくれる研究開発者(大学、企業等)を支援するものであり、優れた研究開発者であれば支援してくれるというものではありません。
その募集に申請するためには、公募される研究事業ごとに主催者側がどういった目標・目的を持っているのか、どういった社会課題のどの段階の研究開発成果を求めているのかを入念に把握するとともに、自己の技術、着想、発展性、構築体制などを客観的にみて、自己の研究開発能力を当該研究事業に適合させるように研究開発を提案する必要があります。そして、その募集に採択されるためには、審査において他の申請者より高い評価を得なければならず、評価者側からの視点において意義のある研究開発を提案する必要があります。研究開発提案書は、「自分が書きたいことを書くのではなく、評価者が書いて欲しいことを書く」のです。
CDIメディカルでは、産学連携による医療製品を開発する企業、共同研究開発をする大学、公的研究機関の皆様への研究開発の推進のための公的研究資金獲得の支援を開始します(「産学連携の研究開発アドバイザリー事業」下記URL参照)。また、医療製品の多くは、医療に上市するために厚生労働省の認可、医療保険の適用を受けなければならず、デジタルヘルス製品、新原理を用いる医療機器や体外診断薬などの製品開発は、上市するために必要とされる性能、要件、水準などの検討に困難が伴います。このような製品開発では、開発初期段階から薬事戦略を建てていく必要があり、産学連携における製品開発においても、薬事などの規制・制度への対応の支援も行います。
世界における医療製品の開発競争は激しさを増しており、世界へと通じる日本発の医薬品、医療機器等の研究開発は将来の日本の産業を支える柱であるとして、AMEDなどの公的研究資金による産学連携開発研究の支援は厳しい国家財政の中でも高水準で維持されています。産学連携による医療製品の開発において、公的研究資金の活用は研究開発を次の段階へと進めるために最重要な手段の一つです。
*:産学連携による医療製品の研究開発の支援は、AMED以外にもJST(国立研究開発法人 科学技術振興機構)や一部自治体においても実施。
産学連携による医療製品の研究開発のイメージ
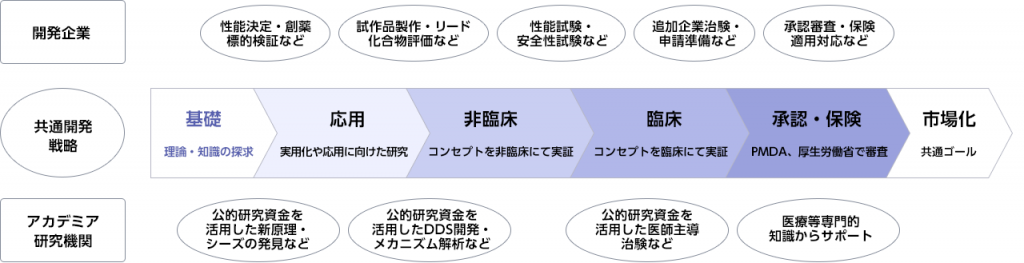
〇 産学連携における研究開発アドバイザリー事業
〇 産学連携による公的研究資金支援事業の例(AMED令和2年度 「医療分野研究成果展開事業(先端計測分析技術・機器開発プログラム)」に係る公募 )
文責:岡野内 徳弥

岡野内 徳弥(株式会社CDIメディカル 主査)
静岡県立大学大学院薬学研究科修了、マサチューセッツ大学ビジネススクール修了。
博士(薬学)、経営学修士。
厚生労働省、独立行政法人国立病院機構、独立行政法人医薬品医療機器総合機構、国立医薬品食品衛生研究所、環境省、法務省、神奈川県を経て、現在に至る。