新型コロナウイルス COVID-19 ワクチンの開発
新型コロナウイルス(COVID-19)に関する緊急事態宣言が解除となって1ヶ月が経ちます。
経済活動も徐々に再開されていますが、東京では連日50人前後の新規感染者が出ていて、ウイルスの脅威が去ったとはいい難いようです。むしろ世界では感染者数の増加は拡大している状況でもあり、世の中が平常化するにはまだまだたくさんのハードルがありそうです。
ウイルスへの対抗策として期待されるものの1つがワクチンの開発ですが、国内でも初のワクチンの臨床試験開始が発表されました。大阪のベンチャー企業アンジェスが開発をしているものです。
–アンジェス株式会社のプレスリリース–https://www.anges.co.jp/pdf.phppdf=DdaV8kN5YuxAj2GdulIHFPOq7iFVvbdT.pdf
遺伝子ワクチンの開発に期待
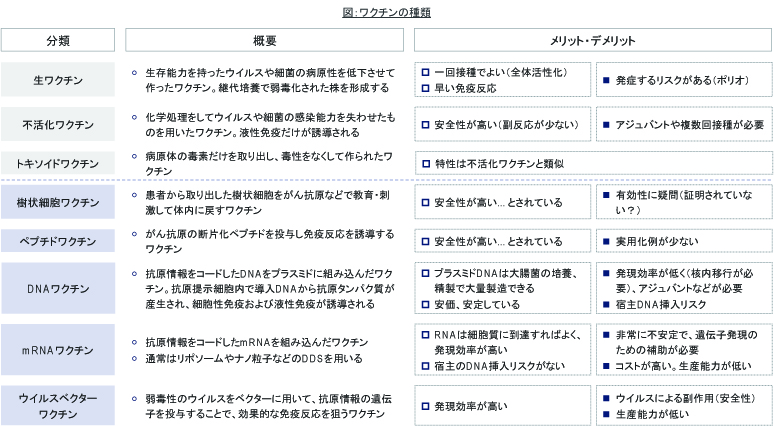
このワクチンは、『DNAワクチン』という、遺伝子ワクチンの一種で、従来のワクチンとは技術的に異なるものです。現在、COVID-19のワクチンとして開発が先行しているものには、英Oxford大/AstraZeneca(ウイルスベクターワクチン)、米Moderna Therapeutics(mRNAワクチン)などがありますが、いずれも遺伝子ワクチンになります。これらはウイルスが持つ遺伝子配列の一部をワクチンとして利用するものです。
従来のワクチンは、病原性のあるウイルスを元々の出発材料としていて、これを鶏の卵で増やしたり毒性を弱めたりする必要があるため臨床試験用のワクチンにするまでに1-2年かかるといわれています。一方、遺伝子ワクチンはターゲットとなる遺伝子配列を製造すればよいので、開発の速度をはやくすることができます。現在、一刻もはやくワクチンが実用化されることが望まれる中で、遺伝子ワクチンは大きく期待される技術の1つだといえます。
ただし、これらの遺伝子ワクチンは、まだ一部の動物薬などでしか製品化例がありません。今回、世界的に多数の開発がすすんだ結果として、技術的な課題が解決され、あるいはアジュバントなどの利用の仕方が工夫され、実用技術として確立するかもしれません。
コロナ禍では、多くの人がリモートワークを余儀なくされて、改めてIT技術の重要性が認識されていています。メディカルの分野でもデジタル技術が注目を集めるところですが、そのような中で、いわゆるバイオテクノロジーであるワクチンの新技術も、危機的状況における技術イノベーションの好事例となることを期待したいと思います。
文責:伊藤 愛

伊藤 愛(株式会社CDIメディカル コンサルタント)
大阪大学大学院薬学研究科修士課程修了(薬剤師)。京都大学大学院医学研究科修士課程修了。
商社、独立系ベンチャーキャピタル、ヘルスケア・バイオベンチャー企業、経営コンサルティングファーム等を経て現職。ライフサイエンス・ヘルスケア分野を中心に、中期経営戦略等、新規事業戦略、海外展開、オープン・イノベーション戦略等、戦略立案から実行支援を含むコンサルティングを実施。