薬機法の改正について(その6)
改正されました薬機法についてのコラム(その6)です。今回は、改正事項として「個人輸入に関する規制等の見直し」について解説いたします。
1.改正事項の背景
医薬品、医療機器などの医療製品を海外から輸入する場合には、薬機法の下でその製品の承認や認証を受けた企業等による輸入が原則です。しかし、医師などが疾病の診断・治療・予防の目的で使用する場合、治験や試験研究に供する目的で使用する場合、医薬品などを学術研究や研究開発の為(宣伝広告は不可)に展示会などで展示する目的で使用する場合、一般の人が個人的に医薬品を使用する(医師の処方せん等が必要)場合などは、医療製品の個人輸入をすることができます。ただし、個人輸入はあくまでも特定の目的で使用する場合のみ認められていることであり、医療製品を日本国内で流通・販売するためには、薬機法の承認・許可を受けなければなりません。
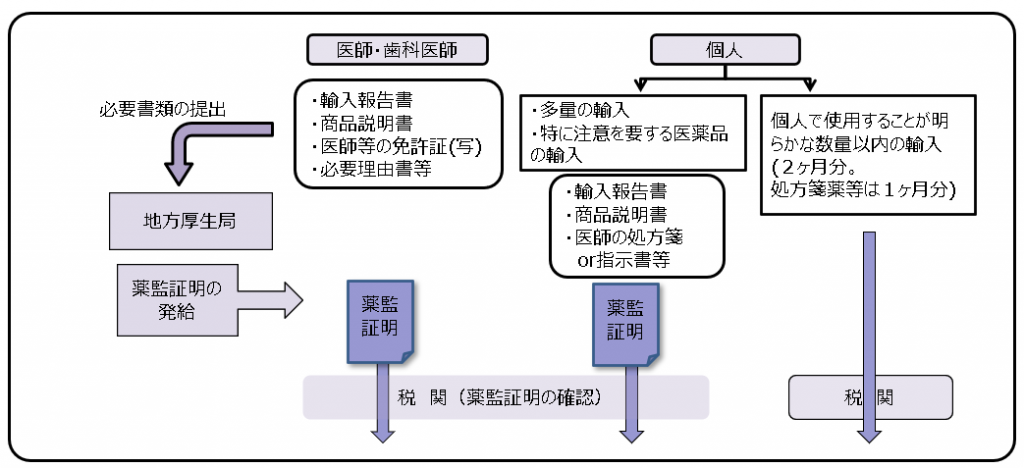
また、このような個人輸入によって医薬品や医療機器が不正に流通・販売されることや偽造薬が国内で流通する恐れがあります。そのため、無許可、無登録品や不良品等が違法に国内に流入することを未然に防いで健康被害を防止する必要があります。このような背景や目的の下、医療製品の個人輸入については、従来、「薬監証明制度」という仕組みで行政通達(通知)*により実施されていました。(下図参照)
薬監証明制度
「医薬品・医療機器等の品質・有効性及び安全性の確保等に関する法律等の一部を改正する法律案の概要」(厚生労働省説明資料より筆者改変)
しかしながら、この制度の下で悪質な違反が起きていたのも事実です。例えば、輸入代行業者が、過去に医師の輸入代行を行った際に取得した医師免許証の写しを本人に無断で使用するという虚偽の申請により取得した輸入報告書(薬監証明)で、米国製の未承認医療機器を輸入し国内で販売していたといった事件が実際に起きております。この事例のような不正行為は詐欺罪(刑法第246条)で刑事告発されました。これには、個人輸入の手続が通知により運用されていたため違反行為に薬機法の罰則を掛けることが困難であったことが理由としてありました。
現在、医療製品の個人輸入の件数は増加傾向にある中、事業者の違反の取締りが薬機法上困難であったことをなどから、取締りを強化するために、通知により運用されていた薬監証明制度が法制化されました。
*:平成27年11月30日付薬生発1130第1号「医薬品等及び毒劇物輸入監視要領について」
2.個人輸入に関する規制等の新たな制度
以上述べました背景により、厚生労働省の通知により運用されていた薬監証明制度が法制化され、以下のような新たな制度となりました。
(1)製造販売の承認若しくは認証を受けずに、又は届出をしないで医薬品、医療機器等を輸入しようとする者は、その輸入について、厚生労働大臣の確認を受けなければなりません。
ただし、日本で承認されている医薬品などで、厚生労働省令で定める数量(医薬品なら使用数量、外用剤なら24個等)以下のものを自ら使用する目的で輸入する場合には確認を受けることを要しません。また、麻薬及び向精神薬取締法や毒物及び劇物取締法などに違反して2年を経過していない者は、輸入確認を受けることができません。
なお、手続きについては、基本的に従来の通知運用の手続きが踏襲されています。
(2)輸入確認の手続については、薬機法に基づく指導・取締りが行われ、違反者には罰則(3年以下の懲役若しくは300万円以下の罰金、又はこれを併科)が適用されます。
(3)その他、個人輸入による未承認医薬品や偽造医薬品の流通などの不正事案に迅速に対処するため、偽造医薬品に関しては、麻薬や覚せい剤犯罪の捜査官である麻薬取締官・麻薬取締員も取締りを行います(犯罪捜査のためではないという制限はあります)。
以上、今回は「個人輸入に関する規制等の見直し」について解説いたしました。次回以降も、それぞれの改正事項について解説していきます。
文責:岡野内 徳弥
本コラムの無断転載を禁止いたします。

岡野内 徳弥(株式会社CDIメディカル 主査)
静岡県立大学大学院薬学研究科修了、マサチューセッツ大学ビジネススクール修了。
博士(薬学)、経営学修士。
厚生労働省、独立行政法人国立病院機構、独立行政法人医薬品医療機器総合機構、国立医薬品食品衛生研究所、環境省、法務省、神奈川県を経て、現在に至る。